Implementation of the PowerPlex® 18D System in a Databasing Laboratory: Transforming Operations to Improve Efficiency
Michigan State Police, Lansing, Michigan, USA
Publication Date: 2011
Introduction
The primary goal of forensic DNA databasing laboratories is the timely development of accurate, reproducible DNA profiles from convicted offender and/or arrestee samples for entry into the National DNA Index System (NDIS). Managers are constantly searching for methods to improve efficiency and decrease cost, and those managers that oversee forensic laboratories are no exception. The processing of standardized DNA samples from convicted offenders or arrestees, hereinafter referred to as database samples, often is responsive to workflow improvements. With the ever-increasing demand for forensic DNA testing, efforts must be made to reduce time and cost. Furthermore, the benefits of improving the DNA database operational process are numerous. Without the timely population of NDIS, investigators may experience an unnecessary delay in receiving probative information related to the crime being investigated, which may delay apprehension of a suspect. Improving the DNA process for database samples will allow personnel to prepare for the seemingly interminable legislative and policy changes such as continual expansion of the collectable population and legal challenges to the retention of database samples. If the DNA database process is improved, resource reallocation also may be appropriate to dedicate additional personnel to the processing of DNA casework, which is, by its very nature, more time-consuming on a per case basis.
Although strategic process improvements often can be implemented to streamline laboratory operations, a technological change is eventually necessary to take process improvements to the next level. Promega Corporation has developed the PowerPlex® 18D System, which will allow significant operational improvements to DNA database sample processing. The development and future implementation of the PowerPlex® 18D System will provide the technological change necessary for laboratories to make large efficiency advancements. In short, the PowerPlex® 18D System allows amplification directly from buccal or blood punches from a Whatman FTA® card in less than 90 minutes (1). Additionally, to improve discriminatory power, two additional STR loci have been added to the original PowerPlex® 16 System loci—D2S1338 and D19S433.
“The new, improved amplification chemistry allows direct amplification of blood or buccal FTA® punches and reduces amplification time to less than 90 minutes.”
Current Challenges For DNA Databasing Laboratories
Legislative changes can increase the number of database samples received by a laboratory, with little time allotted to ramp up operations. In addition, legislative changes may affect laboratory operations in manners unexpected by legislators, lab managers or even database scientists. For example, the effect of inclusion of arrestee database samples cannot be projected by merely estimating the additional number of samples that would be submitted to the laboratory. The most time-consuming effect of adding arrestees to collection legislation is often the increased time needed at sample reception: that is, determining if the sample was appropriately submitted and under what circumstances (convicted offender versus arrestee).
Resource allocation continues to plague forensic laboratories across the nation, and DNA databasing sections are no exception. Pairing shrinking budgets with increasing database sample numbers creates a situation in which continual process improvements are essential. The sample collection type can assist with process improvements, as well as implementation of standardized collection substrates, such as Whatman FTA® cards. Automation has been introduced and successfully implemented into many DNA database labs, but for some labs increased sample numbers still outweigh these improvements. Ultimately, finding the proper balance to produce high-quality data in a rapid but cost-effective manner is the goal for all DNA databasing labs.
The PowerPlex® 18D System
Operational Changes to the Current Procedure
The DNA processing procedure employed by the Michigan State Police for database samples currently consists of the following steps:
- Sample Cutting
- Purification
- Amplification
- Setup and Detection
- Data Review and Import into CODIS
With implementation of the PowerPlex® 18D System, the purification step can be eliminated completely (Figure 1). The new, improved amplification chemistry allows introduction of blood or buccal punches directly into the amplification master mix, and the amplification step is reduced to less than 90 minutes for a 27-cycle amplification. When processing large numbers of standardized samples in a production-line manner, as is done in many DNA databasing laboratories, the time reductions experienced will have a significant impact on sample-processing efficiency. This processing time reduction also changes the timeline for a “rush” sample, as one sample can be processed, reviewed and uploaded into CODIS in approximately 2 hours. The operational changes made possible by the PowerPlex® 18D System are so substantial that resource allocation and sample prioritization must be re-evaluated.
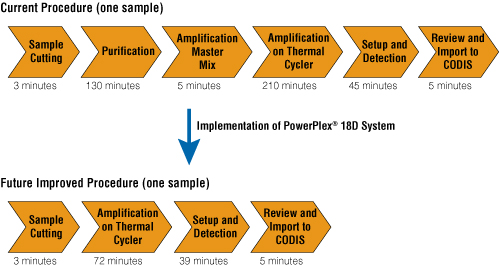 Figure 1. A comparison between the current DNA database procedure and the improved procedure that can be realized with implementation of the PowerPlex® 18D System.
Figure 1. A comparison between the current DNA database procedure and the improved procedure that can be realized with implementation of the PowerPlex® 18D System.
Most notably is the removal of both the DNA Purification and Amplification Master Mix steps, which could allow processing to uploading of a sample to be complete in approximately 2 hours. For the improved procedure, the sample cutting is added directly to the Amplification Master Mix. Manufacturer's recommended analysis run time for the CC5 ILS 500 allows a time reduction in the Setup and Detection step.
Feasibility Studies
The potential for process improvements must be weighed appropriately with the quality of the amplification chemistry being considered. An improvement to the process can be quickly dismissed if it is accompanied by a decrease in data quality. Basic verification testing has been performed on the prototype PowerPlex® 18D System with very promising results. The system was used to successfully generate DNA profiles from both buccal and blood punches of convicted offender samples collected by law enforcement agencies. Additional tests, some of which are summarized below, were performed to challenge the system to perform under conditions conducive to increased efficiency.
Thermal cycler parameters for the prototype PowerPlex® 18D System were based on Promega Corporation’s recommendations, with the number of cycles ranging from 25 to 28. A freshly prepared buccal sample on a Whatman FTA® card was used to determine the optimal cycle number. At 25 and 26 cycles, the signal strength was lower than desired. At 28 cycles, many peaks were at the saturation point and were difficult to size properly. As a result, 27 cycles was selected for the remaining experiments because it gave the best overall performance with no samples rejected due to low signal strength or uninterpretable saturated peaks.
A master mix of amplification reagents was tested for stability at both room temperature and standard refrigeration. The master mix was stored, protected from light, in both conditions and tested at several time intervals. After 3 days of storage, the mix stored at room temperature showed a noticeable loss of performance, while the mix stored at 2–8°C did not show any loss of performance after 6 days. The CC5 ILS 500 size standard also was tested for robustness. A detection master mix was created with Hi-Di™ formamide and stored under the same conditions as the amplification reagents. After 8 days of storage, there was no discernable loss of performance for either storage condition. The results of these studies indicate that great potential exists to premake mixes of both amplification reagents and size standard/formamide. The successful short-term storage of these premixes offers an area for efficiency improvement whereby the mixes can be made in large quantities in a predetermined time frame. This would reduce daily preparation time and could improve quality by reducing the potential for human error during the daily mixing of reagents.
To test the prototype PowerPlex® 18D System with blood punches, several whole blood samples were spotted on Whatman FTA® cards, then amplified. Although a usable profile was obtained without any modification to the manufacturer’s protocol, minus A artifacts were observed. These artifacts were eliminated with an increase in the final extension time, from 10 minutes to 20 minutes. The minus A artifact was not observed in the buccal samples; however, the additional 10-minute extension may need to be considered for blood samples. The PowerPlex® 18D System offers such a substantial time reduction for amplification that increasing the final extension time to improve data quality for blood samples should be considered based on the results at each individual laboratory.
The final study conducted was a side-by-side comparison between the prototype PowerPlex® 18D System and the PowerPlex® 16 System. Although the failure rates were congruent, reconsideration of appropriate cycle numbers and/or increased injection times may generate acceptable profiles for many of the failed samples from the PowerPlex® 18D System. Additionally, expansion of the PowerPlex® 18D System allelic ladder will require fewer off-ladder allele confirmation runs, greatly reducing the number of standard reruns. Therefore, the expectation to reduce the rerun rate with the PowerPlex® 18D System is reasonable and should be pursued further during internal validation studies.
Moving Forward With the PowerPlex® 18D System
Final product development is complete, and the PowerPlex® 18D System was recently released for commercial use. Timely completion of the developmental validation is absolutely necessary to thoroughly evaluate the system and determine the feasibility of its use to develop DNA profiles intended for entry into NDIS. Ultimately, the developmental validation must demonstrate a reliable and robust system that meets NDIS approval prior to its use in DNA databasing laboratories.
When considering the practicality of the PowerPlex® 18D System for your laboratory, the real challenge is the re-evaluation and redesign of laboratory operations to most effectively utilize existing resources. Several of the critical self-evaluation questions that should be asked and considered include the following:
- How much cost savings will be realized when purification/extraction reagents are not needed
- With a reduced processing time, should personnel reallocation be considered?
- Should arrestee samples be given priority for testing? What instances warrant single-sample analysis in 2 hours?
- With saved hands-on time for the analyst, is additional educational outreach for the collection agencies needed? Could collection compliance and sample collection technique be improved through outreach efforts?
- What necessary databasing projects have been delayed due to lack of resources?
With the proper evaluation and implementation of this technical improvement, a manager in a DNA databasing laboratory may have a new tool in the toolbox to tackle the challenging request to “do more with less”.
Conclusion
The PowerPlex® 18D System provides a technological advancement that may propel DNA database laboratories into an improved operational state that would have been unachievable with currently available amplification chemistries. The most notable improvements gained from the PowerPlex® 18D System include the ability to amplify database samples without performing any purification steps and a decreased amplification time of less than 90 minutes. The prototype PowerPlex® 18D System generated DNA profiles with minimal artifacts and exhibited good overall allelic and locus balance. The system was robust enough to allow reagents to be mixed and stored ready for use straight from the refrigerator. With all of these combined benefits, significant operational improvements could be realized by utilizing the PowerPlex® 18D System in a DNA databasing laboratory.
Acknowledgments
The Michigan State Police appreciates the invitation from Promega Corporation to evaluate the PowerPlex® 18D System. Their continuous technical support during this project was absolutely essential for its success.
Many thanks to Donald Yet, CODIS Unit Supervisor, for his assistance with the laboratory testing and evaluation of the PowerPlex® 18D System data.
Every member of the CODIS Unit, whether they realize it or not, contributed to the successful feasibility testing, and I am thankful to them all.
Reference
- PowerPlex® 18D System Technical Manual #TMD031, Promega Corporation.
Related Products
How to Cite This Article
Scientific Style and Format, 7th edition, 2006
French, J. Implementation of the PowerPlex® 18D System in a Databasing Laboratory: Transforming Operations to Improve Efficiency. [Internet] 2011. [cited: year, month, date]. Available from: https://www.promega.com/resources/profiles-in-dna/2011/implementation-of-the-powerplex-18d-system-in-a-databasing-laboratory/
American Medical Association, Manual of Style, 10th edition, 2007
French, J. Implementation of the PowerPlex® 18D System in a Databasing Laboratory: Transforming Operations to Improve Efficiency. Promega Corporation Web site. https://www.promega.com/resources/profiles-in-dna/2011/implementation-of-the-powerplex-18d-system-in-a-databasing-laboratory/ Updated 2011. Accessed Month Day, Year.
Contribution of an article to Profiles in DNA does not constitute an endorsement of Promega products.
PowerPlex is a registered trademark of Promega Corporation.
FTA is a registered trademark of Flinders Technologies, Pty, Ltd., and is licensed to Whatman. Hi-Di is a trademark of Applera Corporation.
Products may be covered by pending or issued patents or may have certain limitations. More information.
All prices and specifications are subject to change without prior notice.
Product claims are subject to change. Please contact Promega Technical Services or access the Promega online catalog for the most up-to-date information on Promega products.